HIP APPLICATIONS
Whether you are addressing damaged tissue in articular cartilage, ligament attachment points, or surrounding soft tissue; consider Wharton’s jelly for Connective Tissue Supplementation to repair or replace the damaged tissue with new healthy connective tissue.
Contact Us
Searching for an approach to address debilitating or chronic hip tissue damage?
When it comes to hips, Connective Tissue Supplementation is a safe and effective option. Have you lost mobility due to damaged tissue in your hip? CryoText™ for hip applications is the place to begin. Let Regenative Labs show how Wharton’s jelly for Connective Tissue Supplementation can be used to repair or replace damaged tissue with new healthy connective tissue.
Wharton’s Jelly Allografts
Intended for homologous use only, their main function is to provide cushioning and structural support and replace missing or damaged tissue in the patient when applied directly to the defect, or injured area, in joints and soft tissues. The allografts are applied via syringe and are typically used in ligament, muscle, or cartilage tears.
Regenative Labs’ resources are divided into the following categories:
Discover
Discover our catalogue of applications showcasing how Regenative Labs’ proprietary products can be used.
View all applicationsSTUDY
Study the latest innovations and the variety of options available to medical specialties.
View PublicationsEXPLORE
Explore the detailed descriptions of our products, their make-up and development through our state-of-the-art process and to identify which products are best suited for your patients’ unique needs.
View all productsCommitment to Quality and Transparency
Regenative Labs is a leader in the field of regenerative medicine. Our commitment to quality and transparency is demonstrated by collecting data from patient outcomes and analyzing it for statistical significance, ensuring physicians make the most informed decision to facilitate the best possible outcomes for their patients.
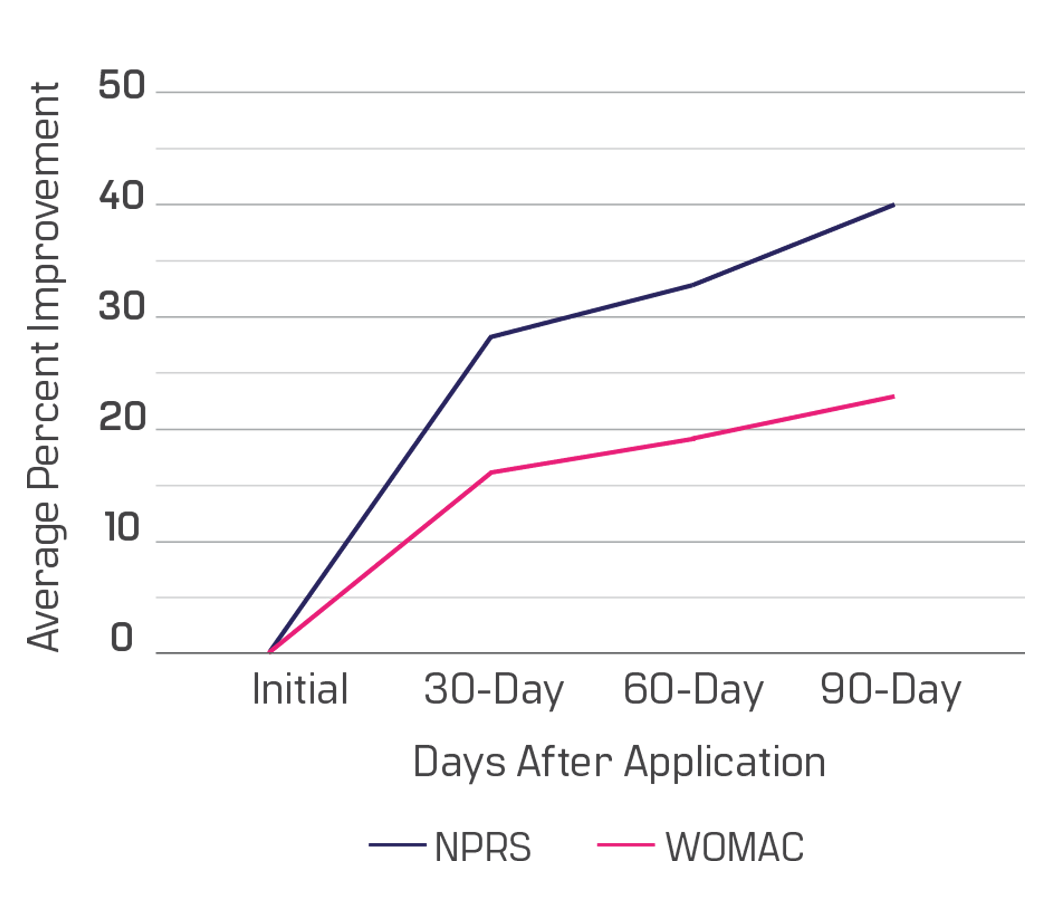
From our retrospective data repository, you will witness improvements beginning at the 30-day mark.
DATA
Data Collection Protocol Approved by the Institute of Regenerative and Cellular Medicine (IRB)
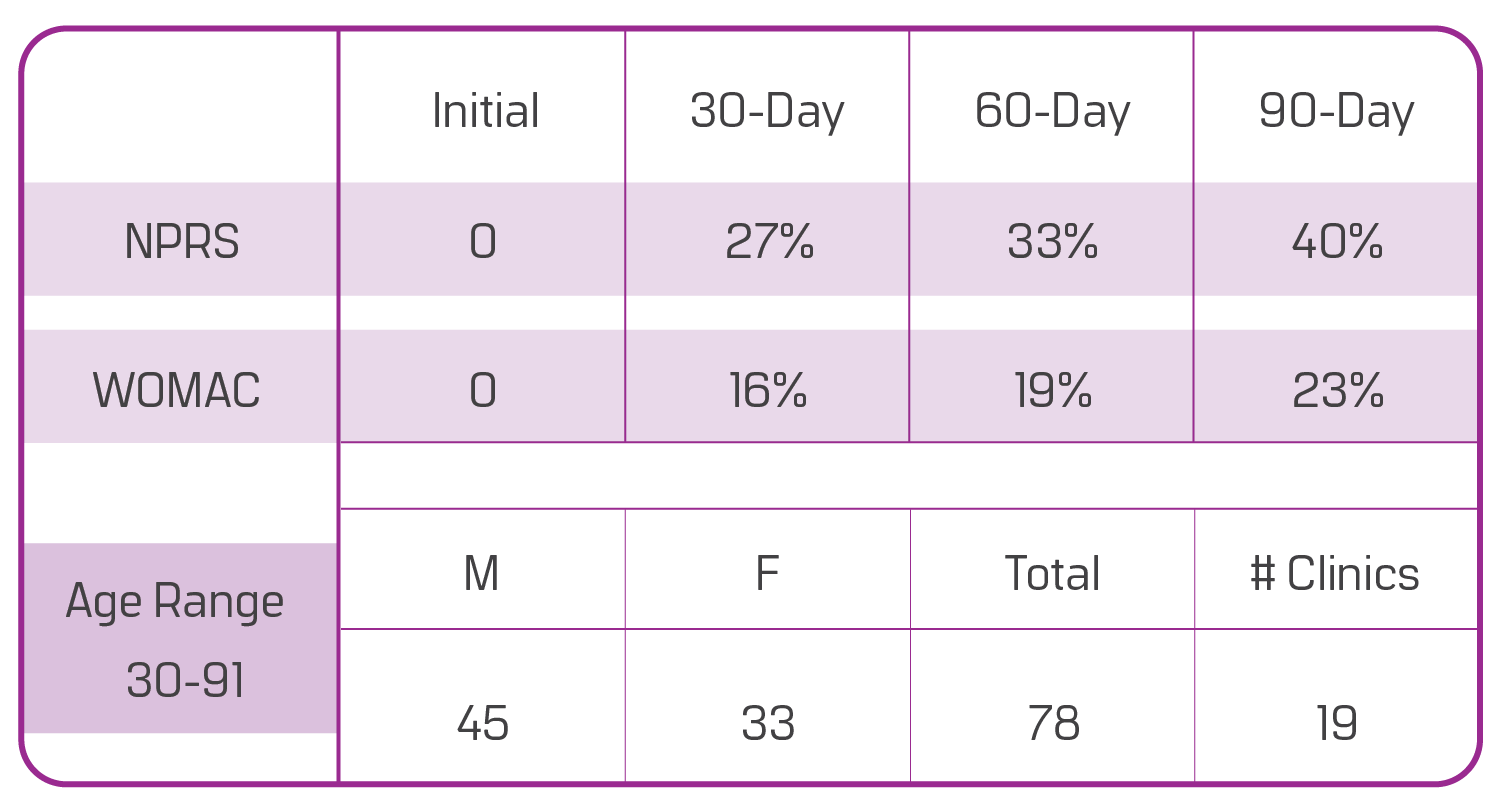
hips
improvements in nprs and womac
This data is based on a sample of 78 patients with structural hip defects from 19 different clinics enrolled in our observational study. There are 45 males and 33 females, aged 30 to 91. Ninety days after the Wharton’s jelly application, patients reported a 40% improvement in NPRS (current pain) and a 23% overall WOMAC improvement (pain/ stiffness/ function). The progress shown is from only one application, although most care providers apply two Wharton’s jelly allografts for this area.
Improving Quality of Life
When we experience pain, our body sends a message that something is wrong. Pain is merely a symptom of an underlying injury. Using steroids or opiates alone to address pain caused by injury masks the issue instead of addressing the root cause.
With connective tissue supplementation, medical providers have an opportunity to address the problem at its source by inserting new, viable connective tissue ECM directly to the site of the breakdown, or defect (via syringe). The patient’s body can use the collagenic superstructure from the newly transplanted Wharton’s jelly as building blocks to fill voids or defects in cartilage beds or other soft tissues.
LEARN About Wharton’s Jelly
About Wharton’s Jelly
Wharton’s Jelly (WJ) was initially characterized in 1656 by Thomas Wharton[1]. Advances in regenerative medicine have increased significantly throughout the past decade. Located between the blood vessels of the umbilical cord and the amniotic epithelium, WJ spans the entire length of the umbilical cord, providing protection, cushioning, and structural support [2,3].
read research articleADVANTAGES OF WHARTON’S JELLY
This connective tissue contains high amounts of extracellular matrix components including collagen types I, III, and V, elastin, and fibronectin [1, 2]. Wharton’s jelly mainly provides cushioning and structural support to the umbilical cord but also contains a natural source of long-chain hyaluronic acid and numerous cytokines and growth factors. Studies have described placental tissues to be “immune privileged” as they rarely evoke an immune response in the body, reducing the risk of adverse reactions [4].
CITATIONS:
1. Kim DW, Staples M, Shinozuka K, Pantcheva P, Kang SD, Borlongan CV. Wharton’s jelly derived mesenchymal stem cells: phenotypic characterization and optimizing their therapeutic potential for clinical applications. Int J Mol Sci. 2013 May 31;14(6):11692-712. doi: 10.3390/ijms140611692. PMID: 23727936; PMCID: PMC3709752.
2. Gupta A, El-Amin SF 3rd, Levy HJ, Sze-Tu R, Ibim SE, Maffulli N. Umbilical cord derived Wharton’s jelly for regenerative medicine applications. J Orthop Surg Res. 2020 Feb 13;15(1):49. doi: 10.1186/s13018-020-1553-7. PMID: 32054483; PMCID: PMC7017504.
3. Deus IA, Mano JF, Custódio CA. Perinatal tissues and cells in tissue engineering and regenerative medicine. Acta Biomater. 2020 Jul 1;110:1-14. doi: 10.1016/j. actbio.2020.04.035. Epub 2020 May 14. PMID: 32418650.
4. Jadalannagari S, Converse G, McFall C, Buse E, Filla M, Villar MT, Artigues A, MellotAJ, Wang J, Detamore MS, Hopkins RA, Aljitawi OS. Decellularized Wharton’s Jelly from human umbilical cord as a novel 3D scaffolding material for tissue engineering applications. PLoS One. 2017 Feb 21;12(2):e0172098. doi: 10.1371/journal. pone.0172098. Erratum in: PLoS One. 2017 Mar 7;12 (3):e0173827. PMID: 28222169; PMCID: PMC5319682.
CryoText™ Product Information
For more information about our Cryotext product, visit the link below.
Pricing and PLACING AN order
For pricing or to place an order, login or setup an account to get immediate access to our MEDNGINE platform.
DON’t HESITATE TO REACH OUT.
If you have any further questions, please contact us. We look forward to speaking with you!