News

Regenative Labs Returned from a Successful PAINWeek 2024
Sep 12, 2024
Regenative Labs had a very successful visit last week sponsoring PAINWeek 2024 in Las Vegas. Our team met numerous physicians,… Read More >

Join Regenative Labs at PAINWeek 2024, September 3-6, at The Cosmopolitan, Las Vegas
Aug 30, 2024
Regenative Labs is excited to announce our participation in PAINWeek 2024, taking place from September 3-6 at The Cosmopolitan in… Read More >

Join Regenative Labs at the Spring Symposium on Advanced Wound Care (SAWC)
May 01, 2024
May 14-18, 2024 SAWC and the Wound Healing Society (WHS) are hosting a spring symposium May 14-18 in Orlando, Florida…. Read More >

Join Regenative Labs at A4M’s 32nd Annual Congress
Apr 17, 2024
METABOLIC CRISIS: FROM SURVIVING TO THRIVING IN THE 21ST CENTURY May 3-5, 2024 A4M/MMI will be hosting its 32nd Annual… Read More >

Homologous Wharton’s Jelly Allografts for Structural Support in Rotator Cuff Tears
Apr 04, 2024
The need for further research in supplementing connective tissue defects was evident given that there are approximately 4.5 million shoulder… Read More >

Regenative Labs, with Advanced Medicine of The Ozarks, Publishes Case Series Demonstrating Utilization of Wharton’s Jelly Allografts in Supplementing Connective Tissue Defects Associated with Tarsal Tunnel Syndrome
Mar 25, 2024
March 25, 2024, Pensacola, FL— Regenative Labs (Regenative), a leading HCT/P manufacturer, announces the publication of a case series demonstrating… Read More >
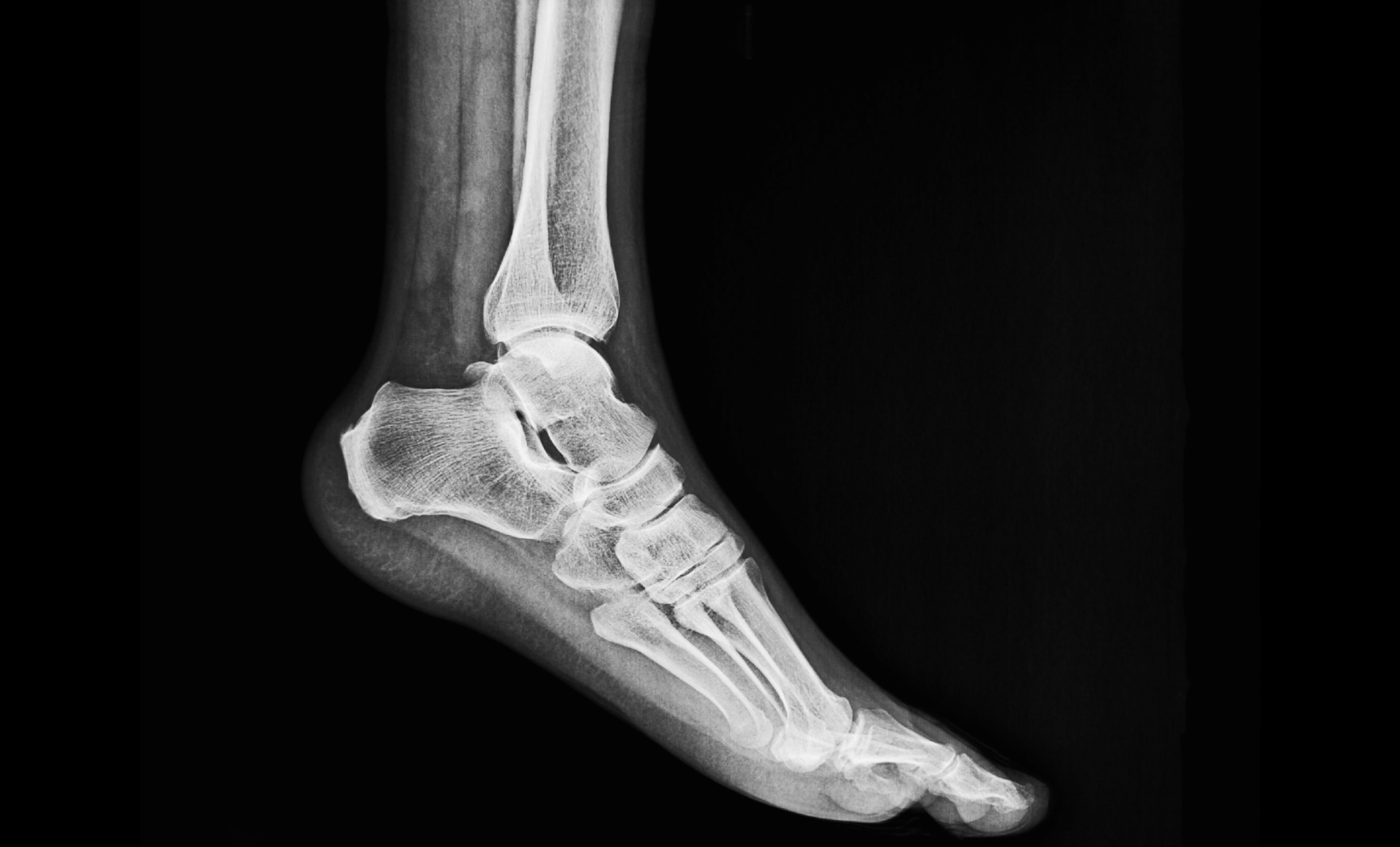
Dr. Lavor Helps Patient Avoid Amputation with Wharton’s Jelly Allograft
Mar 13, 2024
Charcot foot, a rare and progressive degeneration of the foot and ankle joints, is an infrequent complication in general diabetic… Read More >

Exploring and Understanding the Role of Wharton’s Jelly Tissue Allografts in TMJ Defects
Feb 20, 2024
Welcome to our latest blog post, where we delve into a retrospective study exploring a novel approach to address defects… Read More >

Research Findings Present Solutions For Knee Degradation
Feb 06, 2024
One of Regenative Labs’ goals is to give patients with articular cartilage defects more options in their quest to restore… Read More >

Career Trajectory of NBA Players Who Suffered from Achilles Tendon Injuries
Jan 23, 2024
One of the biggest overlooked areas for professional athletes is the importance of the health of connective tissue. The regenerative… Read More >